CURE GM1 CATALYST
Don’t miss all that is happening in our community and the broader rare disease community! There are many important opportunities to engage and to make a difference! Read on to learn more on continued progress and help build momentum.
Table of Contents:
COMMUNITY ENGAGEMENT
ADVOCACY
BIOTECH NEWS
FUNDRAISERS
ANGELVERSARIES & BIRTHDAYS
A Decade of Hope: Celebrating the Cure GM1 Foundation 10th Anniversary
Dear GM1 Community,
Ten years ago this month, on April 17, 2015, the U.S. government approved the Cure GM1 Foundation to be the first and only 501(c)(3) entirely dedicated to GM1 gangliosidosis research, drug development and awareness. Over the course of a decade, our efforts have grown significantly. What began as a more personal search for hope became a global movement that has transformed the landscape for this rare disease.

As we mark this milestone anniversary, I reflect on this remarkable journey. When we started, the words “GM1 gangliosidosis” were mostly unknown, even in the healthcare sector. It took several years to receive my daughter Iris’s diagnosis. I remember asking the doctor to write down the words so I could look up the definition. At that time there was no treatment, very little research, and virtually no hope. Families like mine faced this devastating diagnosis with no treatment options and little support.
Today, that reality has changed. Through the collective effort of families, researchers, clinicians, and supporters, we’ve helped forge and support pathways where none existed before. Dozens of children received chances in clinical trials. One clinical trial is still enrolling newly diagnosed children and a new trial is near. And most importantly, families who once felt alone now find themselves part of a global supportive community.
Our journey over these ten years has been marked by significant milestones. From funding the first gene therapy preclinical research in our founding year to launching our foundation-led Enzyme Replacement Therapy (ERT) project in 2024. We’ve had many projects in between which have led to increased awareness and activity for GM1. We are steadfast in our commitment to accelerating possible treatments and supporting the GM1 community.
In this special anniversary edition of our newsletter, we celebrate not just how far we’ve come, but the countless individuals who made it possible – including you.
The path ahead still holds challenges, but we face them with the confidence, consistency and determination born from a decade of progress and knowledge.
Together, we can accomplish so much. Every single moment, we are closer to our ultimate goal: an approved treatment for GM1. Your continued support and engagement is critical to our future success.
With gratitude and hope for all our future work together,
Christine.
Community Engagement
Some Highlights of our Work

What’s New with the Cure GM1 foundation?

Welcome Aubreigh , Social Media Coordinator
Aubreigh Duits is a senior at the University of North Carolina at Charlotte, graduating in May 2025 with a degree in Business Administration, specializing in Marketing. With hands-on experience in content creation and campaign management, she contributes to crafting clear, engaging communications that build community and foster meaningful connections. As a volunteer at Cure GM1, she is excited to contribute to impactful initiatives and make a difference through strategic digital outreach.
Join the Cure GM1 Foundation Board of Directors
We are grateful for the contributions of BingYune Chen who is stepping down from our board of directors. We wish all the success to BingYune in his endeavors.

“I also want to express my deepest gratitude to each of you for your trust, collaboration, and willingness to explore new ideas. I have learned so much from this experience, and I look forward to seeing the continued progress and success of Cure GM1” – BingYune Chen.
Our mission continues and we’re seeking a new board member. Help Cure GM1 find a skilled professional to join our board of directors. If you would like to see the job description and application, please email info@curegm1.org
Michael Carter’s Story
Learn Michael Carter’s story, who lived with GM1 gangliosidosis. Michael was diagnosed with type 1 and never had the opportunity to experience childhood milestones like walking, talking, and attending school. His mom’s message is powerful: no parent should have to hear that their child has a disease with no treatment or cure.
Read Michael Carter’s full story to learn how your support makes a difference in the fight against rare diseases.

In Loving memory of Milah
It is with deep sadness that we share the news of Milah’s passing. As our community mourns this profound loss, we remember the brightness she brought into this world.
Though GM1 prevented Milah from reaching typical childhood milestones, she communicated in her own special ways. Through all the challenges, Milah remained a happy baby who rarely cried.
As we honor Milah’s memory, we renew our commitment to supporting families facing GM1 and advancing research toward treatments and cures. She will continue to inspire our work and strengthen our resolve to ensure no parent has to hear that their child has no chance. Our hearts are with Milah’s family during this difficult time. Read Milah’s Story

Mother’s Day Tribute to GM1 Caregivers
This Mother’s Day, we want to celebrate the extraordinary moms caring for children with GM1 gangliosidosis. They show incredible strength, love, and dedication every single day.
We invite all family members – husbands, grandparents, siblings, and children – to help us create a special tribute to these remarkable mothers. Please send us:
- A favorite photo of the GM1 mom in your life
- A brief quote or message sharing what makes her special and how she inspires you
Your submissions will be featured in a heartfelt tribute to these extraordinary women who show unwavering love and courage every day.

Email your photo and message to tayara@curegm1.org with the subject line “GM1 Mother’s Day Tribute”. Submission Deadline: April 30, 2025.
Let’s come together to honor our incredible mothers and show them just how much they mean to all of us in the GM1 community.
This is the last call to join Our State Proclamation Campaign!
So far we have 11 requests for proclamations submitted for 2025! Join our effort to make history by getting May 23rd, 2025 officially recognized as GM1 Gangliosidosis Awareness Day in as many states as we can!
Your state’s proclamation helps bring greater awareness and increased visibility to our cause. Ultimately, it leads to greater support and opens pathways for increased funding. We’ve prepared all the materials you need and step-by-step guidance. Many states require submissions weeks in advance, so this is our last call!
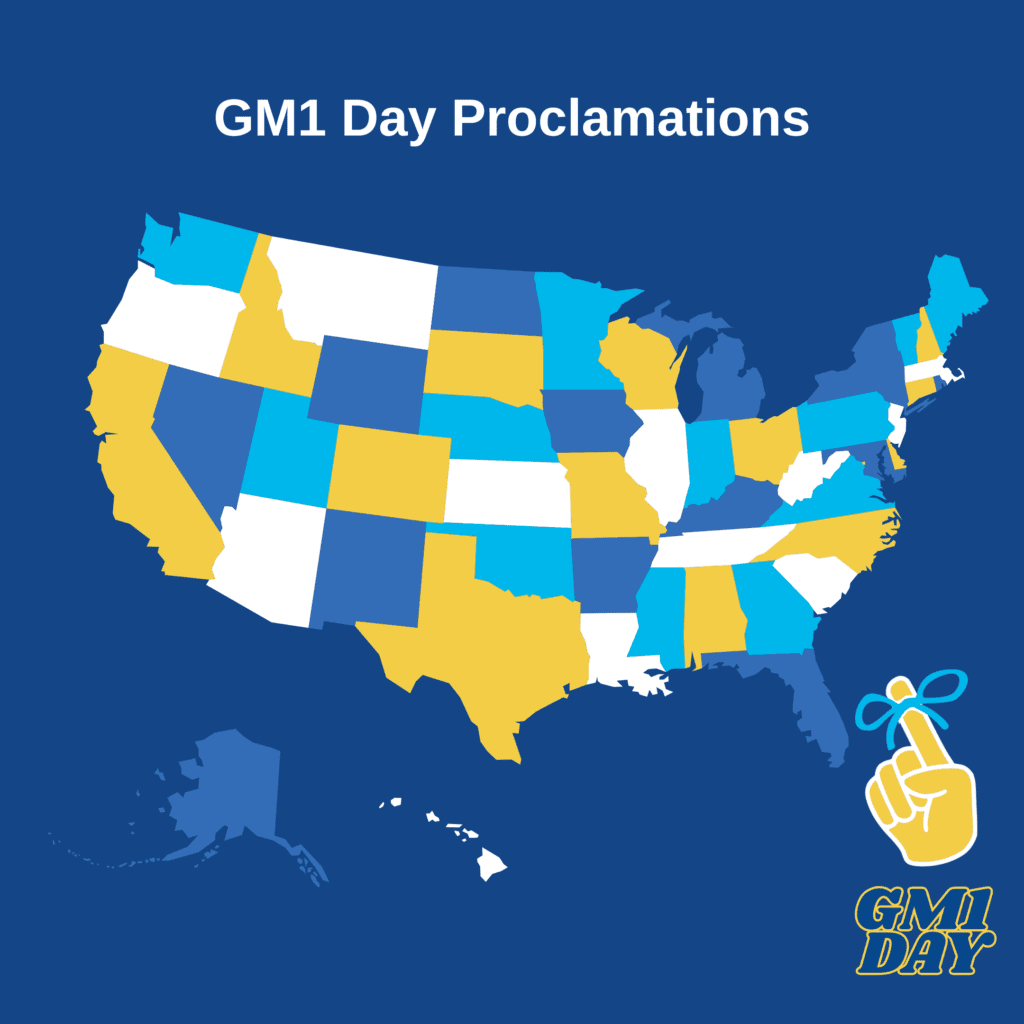
Get in the Press: Now is also the time to reach out to your local press to request a story to be ready for GM1 Day in May. We have all the material ready for you.
Want to help? Email tayara@curegm1.org with “GM1 Proclamation – [Your State]” in the subject line. Together, we can amplify our voice and make a real difference for the GM1 community!
Get Your GM1 Day Merch
We have T-shirts, sweatshirts, and more in a variety of colors and sizes available!
Upcoming Lysosomal Storage Disease Data Sharing Workshops
Don’t miss the remaining sessions in the Lysosomal Storage Disease Data Sharing Workshop series hosted by the Orphan Disease Center (ODC) and the Critical Path for Lysosomal Diseases (CPLD). These virtual workshops are particularly valuable for the GM1 community as they focus on improving data sharing for rare disease research.
Upcoming Dates:
- Session #3: Monday, April 28th (11am-1pm EST) – Case Studies on Retroactive Data Sharing
- Session #4: Monday, June 2nd (11am-1pm EST) – Data Sharing Collaboratives
These workshops will help create resources for registries and natural history studies, empowering our community with tools for sharing critical data. Even if you can’t attend live, registered participants will receive recordings and materials. This initiative directly supports efforts to better understand conditions like GM1 through collaborative research.
Cure GM1’s own data sharing efforts are critical to deepening the understanding of GM1.
Check out the Mother’s Day Gear!
Show Your Support for gM1 Awareness
Mother’s Day Merch
Flower Power Fundraising
Get flowers for the moms in your life and support GM1 research at the same time! We’ve partnered with Flower Power Fundraising to get you access to amazing deals on flowers just in time for Mother’s Day.


See’s Candies
All through 2025, you’ll help the Cure GM1 Foundation continue our work by buying chocolate!
Start with Mother’s Day – With so many available options, your gift is sure to be a hit.
Minted
Mother’s Day has always been reserved as a special day to show the moms in your life how much they mean to you. What better way to do it than with a premium Mother’s Day card?
Use code FUNDRAISECGM1 and you’ll get a 20% discount on cards and prints. The Cure GM1 Foundation will receive 10% of every purchase!

REGISTRATION IS OPEN for 2025 GM1 Community Conference!

Interested in volunteering or helping with the event? We’d love your support! Email info@curegm1.org to get involved. Stay tuned for more details!
Keynote Speaker Announcement!
Registration to our Annual Conference is open! This two day hybrid event will take place in Irvine, CA, on August 7-8th. Don’t miss this unique opportunity to connect with other GM1 families, companies, and researchers in person!
We are thrilled to share Amanda Griffith Atkins, author of How to Handle More Than You Can Handle, as our keynote speaker. Amanda Griffith-Atkins, MS, LMFT, is a licensed marriage and family therapist and founder of Amanda Atkins Counseling Group in Chicago. She is passionate about helping parents of disabled children find support and community. You can follow Amanda’s social media here.
Register here: https://www.curegm1.org/2025-international-gm1-community-conference/


Interview for the National Human Genome Research Institute (NIH)
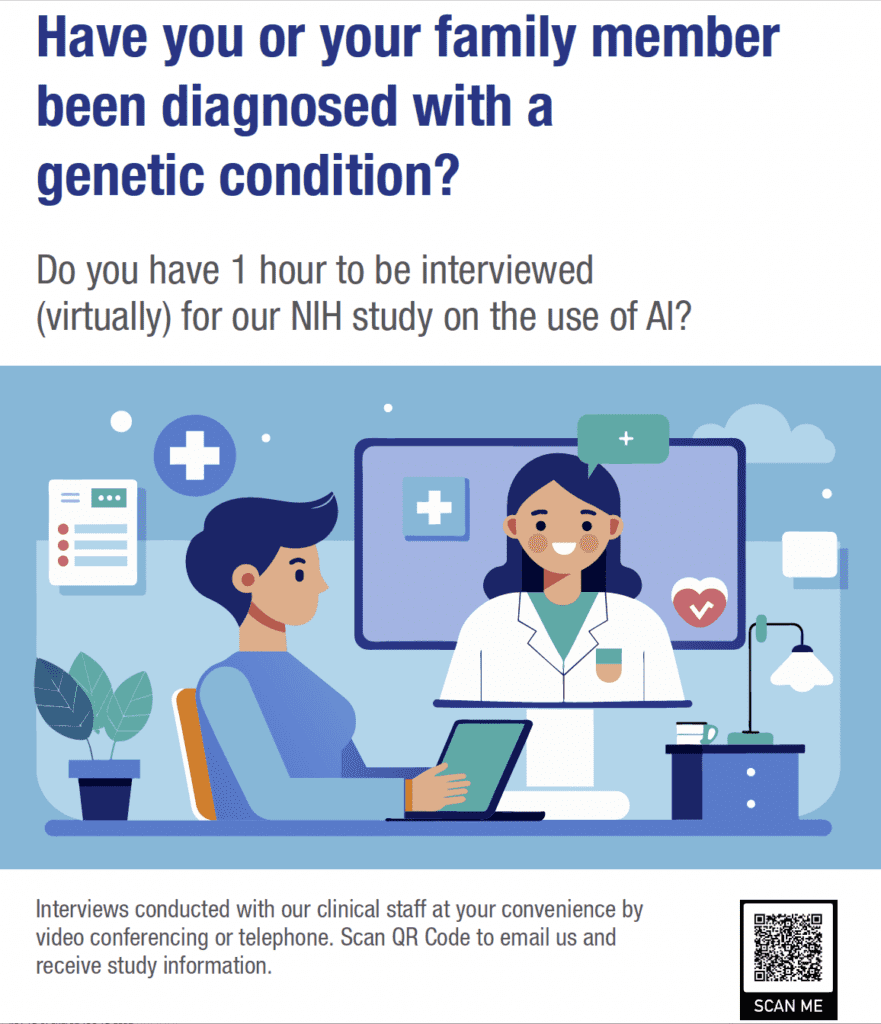
A group at the National Human Genome Research Institute (NIH) is conducting interviews with people who have been diagnosed with genetic diseases, such as GM1. This is a zoom call that could be recorded and analyzed using AI. If you are interested in learning more, you can reach out directly to the group. The researcher’s name is Rebekah and she works with Jean Johnston and Dr.Tifft at the same center, but this is not associated with Dr. Tifft or Jean. This research is a sub study of a larger study: Observational Study of Advanced Data Analytics in Genetic Conditions
Study Contact: medicalgenomicsunit@mail.nih.gov
Advocacy
ASGCT Letter
The American Society of Gene & Cell Therapy (ASGCT) is supporting robust National Institutes of Health (NIH) appropriations. They presented a collective letter to Members of Congress and their staff to advocate for critical research funding in cell and gene therapies.
Cure GM1 has cosigned this letter together with other organizations. ASGCT remains committed to advancing this important initiative and appreciates your collective voice in supporting our shared mission. For any questions about this sign-on letter or our ongoing advocacy efforts, please contact our Chief Advocacy Officer, Margarita Valdez Martinez (mvaldez@asgct.org).
Congress to cut Medicaid Program – Act Now!
Congress is considering massive cuts to the Medicaid program, putting health care access at risk for millions of people living with a rare disease. NORD is mobilizing advocates and fighting for our community, but we need your help!
First, take action by contacting your lawmakers – It takes one minute!
Second, storytelling is the most impactful way to advocate. Congress needs to understand what is at stake for our community. If you’d be willing to share your Medicaid story to help us advocate, please contact stories@rarediseases.org
Critical Rare Disease Funding at Risk: Take Action Now
Congress is currently navigating complex budget decisions that directly impact rare disease funding, with concerning developments including a 57% cut to the Department of Defense’s medical research program and decreases for NIH and other health agencies.
The current continuing resolution expires March 14, with proposed cuts threatening programs vital to rare disease communities. As Congress moves toward FY2026 planning and potential budget reconciliation with significant Medicaid reductions, your voice is crucial.
The rare disease community must act now to protect essential research funding and healthcare coverage by contacting representatives through the provided action alerts to explain why these programs are lifelines for patients and families affected by conditions like GM1 gangliosidosis.
Update on the Rare Pediatric Disease Priority Review Voucher Program
The fight continues to reauthorize the Rare Pediatric Disease Priority Review Voucher (PRV) program. Senators Wyden (D-OR) and Sanders (I) recently brought the Bipartisan Health Care Act to the Senate floor seeking unanimous consent. This critical legislation includes reauthorization of the PRV program, which has already led to innovations helping 47 rare pediatric indications and benefited more than 200,000 patients. Despite its proven track record (with more than half of PRVs granted in just the past four years and over 90% awarded to treatments for conditions with no previously approved therapies), the motion faced an objection from Senator Rick Scott (R-FL).
What can you do? The Give Kids a Chance Act (H.R. 1262) remains our best path forward to restore this vital, cost-neutral program that incentivizes manufacturers to develop treatments for rare pediatric diseases.
Please urge your members of Congress to support this legislation today
Advocacy Events and Opportunities
The Rare Bootcamp – connect with patient advocacy peers and learn from experts
The Rare Bootcamp is a three-day in-person forum, in Cambridge (MA), that provides parents and patient advocates seeking to develop rare disease therapies an opportunity to connect with their peers and to learn from rare disease drug development experts.
It will be held on April 15-17: Learn More. If interested, use this link to get on the bootcamp mailing list and receive registration information.
Biotech News
NIH Expands Support for Ultra-Rare Disease Research
Research Progress: The NIH’s Ultra-rare Gene-based Therapy (URGenT) Network has announced two new research opportunities for clinical trials of gene-based therapies for ultra-rare neurological diseases. This program specifically targets conditions affecting fewer than one in fifty thousand people, offering support to advance treatments from late-stage pre-clinical development into first-in-human clinical testing.
The impact of this program could be transformative for the rare disease community. Around 7,000 known rare and ultra-rare diseases affect 30 million people in the U.S., with approximately 45% being neurological disorders. Most significantly for communities like ours, 85% of rare and ultra-rare diseases are single gene disorders, making them excellent candidates for gene therapy.
The URGenT program represents a crucial funding pipeline that could accelerate the development of treatments for conditions like GM1 gangliosidosis by providing the resources needed to bridge the gap between promising laboratory research and actual clinical trials in patients.
For more information, learn more here.

New Leadership at the FDA: Dr. Marty Makary Confirmed as Commissioner
The Senate confirmed Dr. Marty Makary as the new FDA Commissioner. The Johns Hopkins surgeon and health policy researcher will lead the agency during a period of significant transition, with ongoing staffing adjustments and a renewed focus on addressing chronic disease. Dr. Makary has built his career as a critic of the medical establishment, bringing attention to important issues like hospital workplace culture and its impact on patient care. He pledged to conduct his own assessment of recent agency staffing changes and maintain the FDA’s scientific integrity. As the rare disease community watches this transition, Dr. Makary’s leadership could influence how the FDA evaluates new therapies, including potential treatments for conditions like GM1 gangliosidosis.
The Director of the FDA’s Center for Biologics Evaluation and Research (CBER) resigns
Peter Marks, M.D, Ph.D., who has served as the Director of CBER at the FDA has resigned his position, effective April 5th. Since he began leading CBER in 2016, he has been instrumental in the push to develop vaccines, biologics, and cell & gene therapies, among many other advancements in the field of medicine. Under his leadership, there has been a large and rapid rise in FDA approved cell and gene based therapies – many of which were developed to improve the lives of people living with rare conditions. As a long time friend of the rare disease community, his resignation is somber, however the impact he has had on the FDA’s approval processes and highlights on innovation will undoubtedly leave a lasting legacy at the agency.

Bipartisan Legislation Introduced to Improve Rare Disease Treatment Development
A bipartisan group of lawmakers has introduced the Scientific EXPERT Act of 2025, designed to help bridge the gap between rare disease expertise and FDA regulatory processes. This legislation would formalize quarterly meetings where medical experts, scientists, patient advocates, and FDA reviewers can collaborate on addressing challenges in rare disease treatment development. With over 90% of rare diseases still lacking FDA-approved treatments, this initiative aims to overcome common hurdles such as small patient populations and limited disease-specific expertise among regulators. The Act would create structured opportunities for sharing knowledge without compromising review integrity, potentially accelerating the development of therapies for the estimated 30 million Americans living with rare diseases.
Fundraisers
Make a fundraiser this year!
With all the recent developments with respect to cuts to biomedical research and the public health infrastructure, the work of Cure GM1 is more important than ever. We cannot sit idly and lose ground. Your support matters most in these challenging times.
Our work with respect to our new enzyme replacement therapy project is moving forward. Your support can help move this program forward to help contribute to an arsenal of tools to treat GM1.
Please join us by making a commitment to having a fundraiser this year.
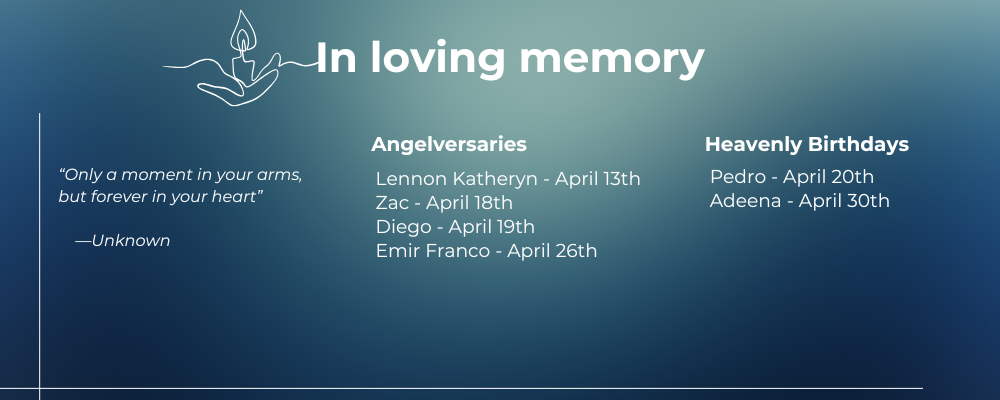
We are also deeply saddened to share the baby Ela passed away on April 2, 2025, just shy of being two years old.

Ways to Give – Your Support Matters
RaiseRight | iGive | Charity Miles | Facebook Fundraisers | Donate |
Set up a Recurring Donation
Visit our Take Action page for more ways to support our community.
Cure GM1 Foundation | PO Box 6890 | Albany, CA 94706 US







