Goodbye June & Hello July.
The CURE Gm1 Catalyst
We have a lot to share this month! First, we are excited to share the final sign-on letter supporting the reauthorization of the Rare Disease Pediatric Priority Review Voucher (PRV) program, with 131 organizations participating. We are highlighting Dr. Sylvia Stockler’s PRO study. Your support and involvement in upcoming focus groups and our virtual conference are vital. Stay tuned for more ways to engage and thank you for your continued dedication to our cause.
Community Engagement
Fundraising
Research and Advocacy
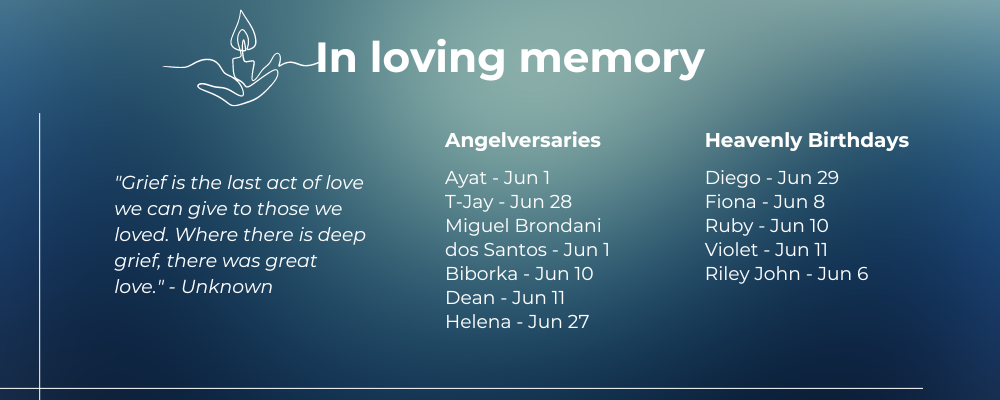
Memorial, Angelversary, Birthdays

Top Reasons to Attend this Year’s 2024 International GM1 Virtual Community Conference
As the agenda takes shape and speakers are confirmed, you might find yourself wondering,
“Is this the year I attend?”
“What sets this year apart?”
“What valuable insights will I gain to become a more effective
advocate for myself or others?”
These are all fantastic questions! To begin uncovering the answers, take a look at our official list…
Hear from leading experts and passionate advocates who are at the forefront of GM1 research. Their stories and insights will leave you motivated and hopeful for the future.
Register now and gain access to all conference recordings. Missed a session? No problem! You can catch up on all the action at your own pace.
Your participation helps amplify the impact of the conference, driving forward the mission to find treatments for GM1 gangliosidosis. Together, we can make a difference!
Enjoy a 15% discount 15CURE on your registration fee until the end of July. Saving money while supporting a great cause? Yes, please!
We invite families to attend the conference at no cost. However, we kindly ask that you sign up so you can receive your swag box and stay informed with all announcements, updates, and more. Don’t miss out on this unique opportunity to be a part of something truly special. Register today and join us in the fight against GM1 gangliosidosis!

Cure GM1 Participated in CIRM Meeting to Advocate for Rare Disease Research Funding
COMMUNITY ENGAgEMENT
On June 27th, Cure GM1 joined other rare disease patient advocacy organizations at the California Institute of Regenerative Medicine (CIRM) meeting. The goal was to secure continued funding for rare disease gene and cell therapy research. Over 1000 people signed the important petition circulated during the event.
This meeting highlighted the vital role of advocacy in bringing funds and attention to rare diseases like GM1. To learn more about this important meeting and to learn more about CIRM, you can see this page and video here. (Watch the end to see our fellow patient advocates speeches and the importance of advocacy to bring funds and attention to our cause.) To learn more about the meeting, visit the CIRM agenda page.
Move for GM1
FUNDRAISING
Move for GM1 is a simple fundraiser that takes place in August. Those who participate log miles walking, running, biking–or anything else–using the Charity Miles App, then share their progress on social channels so others can donate. It’s an easy, fun way to make a difference advocating for those who have GM1 and raising money for Cure GM1.

Earn Donations Through Shopping Using Raise Right
FUNDRAISING
If everyone in the GM1 community used RaiseRight, it could generate tens of thousands of dollars for our cause every year. This is a huge potential impact, for minimal effort. So don’t wait, start using RaiseRight today!

Facebook Fundraiser
FUNDRAISING
Do you have a special day coming up or want to celebrate a loved one’s special day? Consider creating a Facebook fundraiser to make the occasion even more meaningful. It’s a great way to honor your loved ones while supporting a cause close to your heart.

Become A Sponsor for 2024 annual conference
FUNDRAISING
Sponsorship covers overhead costs and keeps the conference free for families. Become a sponsor of the 2024 conference today to support patient advocacy, awareness, and community. Contact us now at info@curegm1.org

UPDATE: Small Molecule Collaboration with Queen’s University
Research and Advocacy
Unfortunately, due to unforeseen circumstances with respect to difficulty with contracting, we have made the difficult decision to no longer proceed with the small molecule project announced last month. We will work with our utmost best effort to continue to identify the best possible projects to advance our critically important mission. We have several projects currently being investigated for possible funding. There will be new information regarding the overall research and clinical trial landscape.

131 Organizations Support Cure GM1 Foundation in Reauthorizing the Rare Disease Pediatric PRV Program
Research and Advocacy
We are pleased to announce that 131 organizations, including the Cure GM1 Foundation, have signed on to support the reauthorization of the Rare Disease Pediatric Priority Review Voucher (PRV) program. This strong turnout is crucial as we push for the program’s renewal before it expires on September 30th. The PRV program plays a vital role in encouraging the development of new treatments for rare pediatric diseases, and its reauthorization is essential for continued progress. Your collective efforts have made a significant impact, and National Organization for Rare Disorders will soon send the final letter to the Senate HELP Committee and Senate Leadership. Now is the time to reach out to your government officials. You can view the full details and the final sign-on letter here. Take Action and Mail your Senators and Representatives here: https://everylifefoundation.org/rare-advocates/take-action/

IRB Approval for xCures GM1 Natural History Pilot Study Funded by Cure GM1
Research and Advocacy
We are excited to announce that the xCures GM1 Natural History Pilot Study, funded by Cure GM1, has received IRB approval! This significant milestone allows us to move forward with our research project, which aims to gather critical insights into GM1 gangliosidosis.
Cure GM1, a 501(c)(3) nonprofit organization located in Albany, CA, USA is proud to announce a unique collaboration with xCures for the development of a new rare disease pilot for an electronic natural history study for GM1 gangliosidosis. Cure GM1 is the only patient advocacy group in the United States entirely dedicated to GM1 gangliosidosis research, advocacy, and development of potential new therapeutics. Read More

Participate in a New Research Study to Develop a Core Outcome Set for Morquio B and GM1 Gangliosidosis
Research and Advocacy
Join a new research study aimed at developing a Core Outcome Set for Morquio B and GM1 gangliosidosis by sharing your experiences and connecting with others in the community. This project gathers the stories and lived experiences of patients and caregivers to create a core list of medical and quality-of-life outcomes. Your participation is vital for making the research meaningful and ensuring future clinical trials align with the community’s interests. Supported by the Priest Family Fund for Morquio B, this study continues previous research on the natural history of these conditions. We invite members from the international community to join our focus group sessions, discuss their experiences, and share what they consider helpful treatment end goals. Participants will be compensated with an online gift card. Limited spots are available, so don’t miss this opportunity to make your voice heard! Find out if you are eligible here. For questions, contact the research team at gm1cos@bcchr.ca.